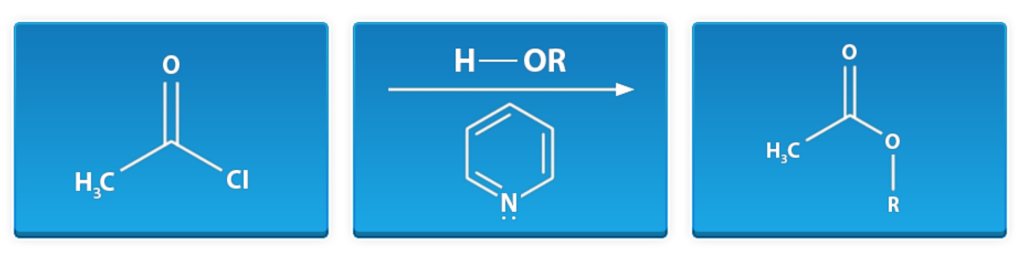
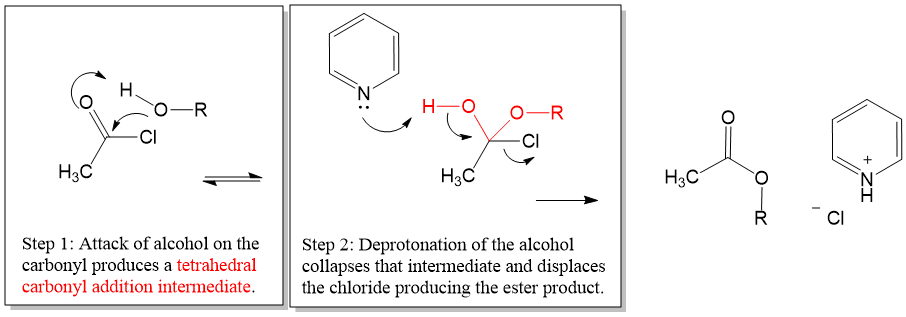
Epoxide Formation
In order to form an epoxide, a electron-rich reagent is required, such as an alkene. Formation of the epoxide occurs in the presence of a peroxide reactant, such as MCPBA or DET. The choice of reagent depends on the stereo-specificity desired from the reaction. MCPBA is used to add the epoxide symmetricaly across the double bond, so that substituents that are cis- or trans- remain in this configuration. The Sharpless asymmetric addition allows the researcher to choose a stereoisomer of DET, referred to as (+) and (-). This reagent allows for the attack of the peroxide intermediate on only one face of the alkene, allowing for the production of nearly pure enantiomeric excess product, generally >98%!
Ring-opening Reactions
Many of you have probably hear of epoxy-glue, which is a very strong binding agent. The process usually involves mixing two reagents and you must quickly apply the mixture to the broken items before the expoxy hardens. One tube will contain the resin, or epoxide, while the other contains the “hardening” agent, which is the nucleophile that will attack the epoxide. In a ring-opening reaction, a molecule such as TETA, which contains 4 amino groups, will attack 4 equivalents of oxirane to produce a complex polymer, which is the basis for a strong glue. Changing the size and complexity of the epoxide can allow for flexibility of strength, thermostability and rigidity!